Structural biotransformations of magnetic nanoparticles in biological environments.
Project Director: Dr. Valentin-Adrian MARALOIU
Superparamagnetic iron nanoparticles (SPIO) have been intensively studied in the last decade. The interest is justified by their properties: larger relaxivity values (improved contrast in Magnetic Resonance Imaging), relatively low toxicity [1]. However, some doubts are hanging over their use due to the long-term toxicity related to the production of toxic free iron during their biodegradation. In this sense, little is still known about recirculation of iron from these nanoparticles in the organism. On the other hand their interactions with the targeted regions in the body have to be understood.
SPIO consist of a ferrite core, magnetite (Fe3O4) or maghemite (g-Fe2O3), surrounded by an organic coating such as dextran, citrate, starch, etc. These nanoparticles have in common their specific uptake by the monocyte-macrophage system and therefore, they are good candidate as MRI biomarkers for the diagnosis of inflammatory disorders associated with high macrophage phagocytic activity.
However, although accumulation of SPIOs in macrophages has been clearly established [2], its intracellular metabolism is not still clear. It is assumed that the particles are completely degraded in the macrophage lysosomal compartment. The organic coating would undergo chemical degradation but little is known about recirculation of iron (from iron core) in the organism. In this sense, it is important to take into account that although iron is essential for life, since it plays an important role in essential cellular functions, free iron is highly toxic to cells due to its capacity to participate in the formation of reactive oxygen species that cause irreversible damage [3]. Moreover, due to the amount of iron oxide particles administered in a MRI session, the iron load per macrophage cell is expected to be high relative to the normal levels. Therefore, maintenance of cellular iron homeostasis is crucial for cell survival and, for this reason, living organisms have developed ferritin to store in a non-toxic form the iron that is not required for immediate metabolic needs. Therefore, an appropriate biodegradation of the iron core of SPIOs is crucial for avoiding any long-term toxicity associated to an excess of free iron.
For example, for the liver, the progressive biotransformation of iron dextran into ferritin or hemeosiderin iron deposits has been explored very recently [4, 5]. These transformations were evidenced using the Selected Area Electron Diffraction (SAED). Using an electron microscope with an atomic resolution, it is possible to illustrate the biotransformations undergone by SPIO from their initial state to ferritin. In CTEM, it is possible to distinguish the differences between ferritin and iron oxide nanoparticles. In the figure below, there is an example of horse spleen ferritin mixed with iron oxide nanoparticles (indicated by black arrows) used as contrast agents for MRI.

References:
[1] IANNONE A., MAGIN R.L., T. WALCZACK, et al., Blood clearance of dextran magnetite particles determined by a noninvasive in vivo ESR method, Magn. Reson. Med., 1991, 22, p. 435 -442
[2] LEVY. M., LUCIANI N., ALLOYEAU D., et al., Long term in vivo biotransformation of iron oxide nanoparticles, Biomaterials, 2011, 32, p. 3988 – 3999
[3] PAPANIKOLAOU G., PANTOPOULOS K., Iron metabolism and toxicity, Toxicology and Applied Pharmacology, 2005, 202, p. 199–211
[4] BRILEY-SAEBO K., BJORNERUD A., GRANT D, et al., Hepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: implications for magnetic resonance imaging, Cell Tissue Res, 2004; 316(3), p. 315-323
[5] GUTIERREZ L., LAZARO F.J., ABADIA A.R., et al., Bioinorganic transformations of liver iron deposits observed by tissue magnetic characterisation in a rat model, J Inorg Biochem, 2006; 100(11), p. 1790-1799
[6] LEVY M., LAGARDE F., MARALOIU A., et al., Degradability of superparamagnetic nanoparticles in a model of intracellular environment: follow-up of magnetic, structural and chemical properties, Nanotechnology, 2010, 21, p. 1 – 11
Structural studies using different electron microscopy technique will be carried out through innovative developments based on electron microscopies down to subnanometric resolution in order to characterize the biotransformations undergone by iron oxide nanoparticles in biological environments.
Task 1: Determination of the initial characteristics of the studied magnetic nanovectors
- Size distribution using CTEM
- Dispersion in liquid media using Wet-STEM
- Crystalline structure of the nanoparticles using SAED and the Fast Fourier Transform of HRTEM images
- EELS spectra on the initial magnetic nanovectors
Task 2: Characterization of the physico-chemical properties of the studied magnetic nanovectors after their insertion in the biological environment
- Size distribution using CTEM
- Changes of crystalline structure of the nanoparticles using SAED and the Fast Fourier Transform of HRTEM images
- Localization of iron nanoparticles using EFTEM by selecting the Fe-L2,3 peak in the EELS spectra of the initial nanoparticles
Project Director: Dr. Valentin-Adrian Maraloiu
Mentor: Dr. Valentin Serban Teodorescu
Task 1: Determination of the initial characteristics of the studied magnetic nanovectors
In a first stage, morpho-structural characteristics of iron oxide nanoparticles (P904 made by GUERBET Laboratories, France) used in MRI as contrast agents were determined. P904 is a contrast agent which is intended to detect in MRI the macrophage cells involved in inflammatory diseases. It consists of a magnetic iron oxide core (maghemite – γ-Fe2O3) which has at the surface an amino alcohol derived from glucose. To determine the characteristics, several electron microscopy techniques were used: a) environmental scanning electron microscopy – Wet-STEM; b) conventional transmission electron microscopy – CTEM; c) high resolution transmission electron microscopy – HRTEM; d) scanning transmission electron microscopy at high angle annular dark field – STEM-HAADF; e) electron energy loss spectroscopy – EELS.
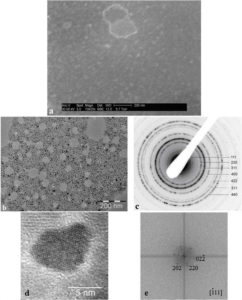
Figure 1. Initial characteristics of P904 contrast agent studied by Wet STEM (a), CTEM (b), electron diffraction (c) and HRTEM (d) with Fourier Transform (e) of HRTEM image.
The behavior of nanoparticles in liquid (the medium in which they are injected into patients) was determined using Wet STEM. An example of a Wet STEM image of a diluted suspension (Figure 1a) confirms the fact that the nanoparticles does not present o strong tendency to flocculation. The iron oxide core of the nanoparticles appears as a white dot in the image; the majority of the nanoparticles from the image has a size between 7 – 10 nm, which corresponds with the core diameter reported by GUERBET. In Figure 1 b - e, there reproduced the results of the study of the nanoparticles using electron microscopy after their deposition and drying on the grid. Electron diffraction pattern (c) made on the nanoparticles from CTEM image (b) shows the maghemite structure. An example of HRTEM image of a nanoparticle (d) and its Fourier transform confirms the good crystallinity of the core.
Task 2.1: Study of USPIO contrast agent nanoparticles injected in healthy mice by different electron microscopy techniques
The aim of the study was to compare the way iron oxide nanoparticles injected into healthy and ApoE KO mice are taken and transformed. Apo E KO mice are genetically modified (the apolipoprotein E responsible with the metabolism of lipids is eliminated) so that they develop faster the atherosclerosis when they are at fat diet regime.
The healthy mice were injected with P904 contrast agent. Before and at different times after administration (1, 9, 15, 42, 66, 95, 127, 176, 238, 283 and 418 days), ex vivo samples from liver and spleen (the main iron processing and storage organs in the body) were taken. These samples were prepared using chemical fixation in order to be observed in the electron microscope. After cutting 50 nm sections at ultramicrotome and their deposition on copper grids, the observations were made at the JEM ARM200F microscope located at the National Institute of Materials Physics.
Before injection (figure 2), cells containing agglomerations of ferritin nanoparticles, amorphous according electron diffraction patterns, can be observed. EELS spectrum made on several agglomerations demonstrate that the atomic concentrations for Oxygen and Iron are close to those corresponding to ferrihydrite (Fe5HO8.4H20).
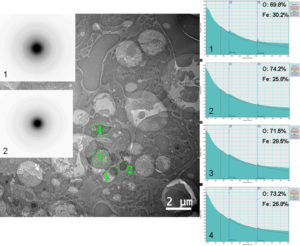
Figure 2. Agglomerations of ferritin nanoparticles in spleen before injection.
In figure 3a, in the CTEM image, it can be observed that nanoparticles are agglomerated in vesicles and that their distribution in tissue is heterogeneous. Nanoparticles are well crystallized (HRTEM image – figure 2c) and, according with the electron diffraction diagram (figure 2b), they keep the crystalline structure of maghemite. EELS spectrum (figure 2e) demonstrate that nanoparticles have the chemical composition close to maghemite (54.91% at O, 45.09% at Fe).
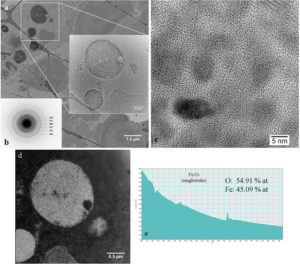
 Figure 3. Study of nanoparticles in spleen at 1 day after administration.
Figure 3. Study of nanoparticles in spleen at 1 day after administration.
In time, these nanoparticles are degraded due to the acidic medium present in vesicles (endosomes or phagolysosomes), the iron released in the cellular environment is taken and stored as ferritin. Ferritin is the protein which stores the iron. It consists in 24 Ft-H polypeptide (MW≈21 kDa) and Ft-L polypeptide (MW≈19 kDa) sub-units , as well as an iron core with a diameter between 2 – 7 nm.
15 days after the administration of the contrast agent (figure 4), vesicles containing maghemite nanoparticles and ferritin nanoparticles can be observed. In region 1, electron diffraction pattern indicates the presence of crystallized nanoparticles with maghemite structure. This is confirmed by the EELS spectrum where atomic concentration ratio for O:Fe is closed to those of γ-Fe2O3. In region 2, the well defined rings in electron diffraction pattern demonstrates the presence of P904 nanoparticles and diffused ring shows the presence of degraded nanoparticles.
Figure 4. Study of nanoparticles in spleen 15 days after injection.
95 days after injection, there are still cells (figure 5 - CTEM image) containing agglomerations of nanoparticles still well crystallized (figure 5 – HRTEM image) having maghemite structure (figure 5 – electron diffraction diagram). EELS spectrum made on such of agglomeration confirms the chemical composition characteristic to maghemite. Outside these agglomerations, in the intracellular environment, ferritin nanoparticles can be found (figure 6 – CTEM image). EELS spectrum made on such ferritin nanoparticles shows that they have a chemical composition (76.79% at O, 23.21% at Fe) characteristic to ferrihydrite (Fe5HO8.4H20). 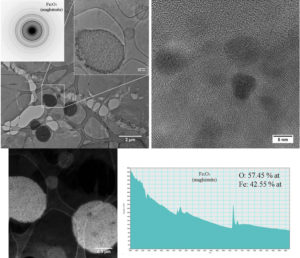
Figure 5. Maghemite nanoparticles present in spleen at 95 days after the administration of the contrast agent.
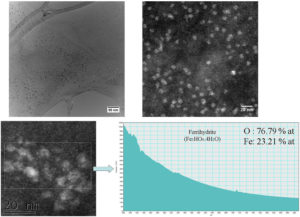
Figure 6. Ferritin nanoparticles present in spleen at 95 days after the administration of the contrast agent.
127 days after intravenous injection, P904 nanoparticles were no longer observed using transmission electron microscopy. In CTEM images (figure 7a-b), it can be observed that there are two agglomerations with different dimensions which contain mostly ferritin nanoparticles according to the informations obtained from EELS spectra. Using a nanometric beam in STEM mode, a chemical map of key elements (iron and oxygen) present in a well defined area (green rectangle in figure 7c) from an agglomeration of nanoparticles was obtained. EELS spectra made on nanoparticles from that agglomeration demonstrate the existence in a limited number of nanoparticles with the chemical composition close to that of the maghemite.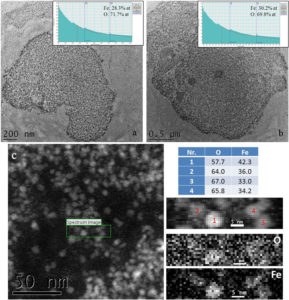
Figure 7. a-b) Agglomerations of ferritins present in spleen at 127 days after injection of P904 agent; c) map of O and Fe on an area into such agglomeration of nanoparticles.
At a longer period of time (238 days) from the administration, P904 nanoparticles were totally biotransformed in ferritin (figure 8).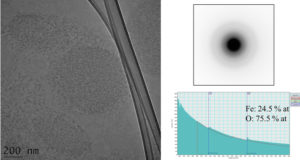
Figure 8. Ferritin nanoparticles present in spleen at 238 days after P904 agent administration.
Observation similar with those presented above were made on the liver samples. There are no differences from the spleen regarding the process of internalization and transformation of the iron oxide nanoparticles into ferritin.
These conclusions were confirmed also by the magnetic measurements made on tissue samples by the team lead by Dr. Veronique Dupuis from the Magnetometry Center of Claude Bernard Lyon 1 University.
Magnetic measurements (figure 9a) showed that the signal of P904 nanoparticles is dominant in the first three months after their injection into the body. Between 95 and 120 days, while the P904 contrast agent is eliminated or biotransformed, their signal decrease, the curves closing to that of the control mice.
Looking carefully at the curve at 120 days (figure 9b), it can be observed the appearance of a peak at 9K and the existence of a double component with a maximum around 70K. The signal in the presence of a weak magnetic field (FC) separates from the signal obtained in the absence of the magnetic field (ZFC) at this temperature, this being a sign of the existence of a maximum in ZFC curve. This maximum corresponds to P904 agent. It should be noted the fact that ferritin peak (T=9K) is so weak that it is clear that it cannot be detected for the days before (1, 9, 95) when this is in minority compared to the signal from P904 signal.
At 176 days after injection, there is no signal from P904 agent (no maximum at 70K) and the curve is close to the one obtained from the spleen of the control mouse.

Figure 9. ZFC / FC curves in spleen at different days from contrast agent injection.
Task 2.2: Study of USPIO contrast agent nanoparticles injected in healthy and ApoE KO mice by two-photon microscopy
This study was made in collaboration with the group lead by Dr. Boudewijn van der Sanden from Grenoble Institute of Neurosciences.
For this study, P904 contrast agent marked with Texas Red Cadaverine fluorophore was injected in healthy and ApoE KO mice. At different time intervals after injection (1 hour, 15 and 40 days), ex vivo samples from the aorta, liver, spleen and kidney were taken. Sections from these tissues were immediately observed using a two-photon microscope LSM 7 MP (Zeiss, Germany) equipped with a 20x water immersed objective immersed (NA 1.0; Zeiss). The excitation wavelength of the pulsed infrared laser (Ti:Sapphire, Chameleon Ultra II; Coherent, UK) was 800 nm.
The observations made showed that, at 1 hour after injection and regardless of mouse type, some of the nanoparticles are already in kidneys from where they will end in the urine. At 15 days after administration, the absence of nanoparticles from the aorta of healthy mice and the accumulation of nanoparticles in macrophage cells present in the atherosclerosis plaque in ApoE KO mice were observed. The presence of nanoparticles in liver and spleen was confirmed at all time intervals, regardless of mice type.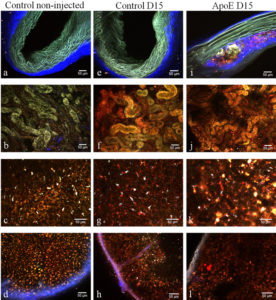
Figure 10. The columns of figure 5 represent three different mice groups: column 1: control non-injected C57bl/6 mice, column 2: injected C57bl/6 mice at 15 days after injection, column 3: ApoE mice at 15 days after injection . The rows are the different ex-vivo two-photon images of the tissues: row 1: lateral views of non-fixed aortic arches, row 2: non-fixed tubules of the cortical area in kidneys, row 3: lateral views of non-fixed liver lobes and row 4: the red pulp (cortical area) of the spleen. (Scale bars, 50 µm).
CONCLUSIONS
Two photon microscopy confirms the heterogeneous distribution of nanoparticles agglomerations in tissues observed by transmission electron microscopy at low magnification.
Comparing the results presented above on normal mice with those obtained on ApoE KO mice, one can notice the fact that there are no differences in the biotransformations process of iron oxide nanoparticles injected into the body. These nanoparticles are internalized by macrophages, they agglomerate in cellular vesicles (endosomes or phagolysosomes) and they are transformed slowly into ferritin.
The electron microscopy observations and the magnetic measurements showed that the initial iron oxide nanoparticles can be found unchanged in cellular vesicles up to 3 months, time in which the organic coating is degraded. The acidic environment present inside the phagolysosomes induces a biotransformation of the crystalline structure of nanoparticles, the majority of them passing from maghemite to ferrihydrite. 6 months after their injection, the iron oxide nanoparticles with the maghemite structure are totally transformed which leads to the formation of a ferritin excess in the studied organs.
The transmission electron microscopy study also demonstrated that the iron excess due to the injected dose of nanoparticles had no toxic effects on the body. The conclusion is supported by CTEM images at low magnification in which it can be observed that the cells which internalized the nanoparticles are not necrosis. This means that the iron is well tolerated by the body even in excess.
The results obtained during this project will be included in two articles as follows:
- the results obtained with the colleagues from Grenoble Institute of Neuroscience will be included in an article entitled „Multiscale investigation of USPIO nanoparticles in atherosclerotic plaques and their catabolism and storage in vivo” which was submitted to Nanomedicine: Nanotechnology, Biology and Medicine.
- the article „Ferritin surplus in mouse spleen 14 months after intravenous injection of iron oxide nanoparticles at clinical dose”, made with the colleagues from Magnetometry Center of Claude Bernard Lyon 1 University was submitted to ACS Nano.
1. V.A. MARALOIU, A. BROISAT, F. APPAIX, D. LEGUELLEC, V.S. TEODORESCU, B. van der SANDEN, M.G. BLANCHIN, “Multiscale investigation of USPIO nanoparticles in atherosclerotic plaques and their catabolism and storage in vivo”, Nanomedicine: Nanotechnology, Biology and Medicine, 12 (1), 191-200 (2016) doi:10.1016/j.nano.2015.08.005
2. A. Tamion; M. Hillenkamp; A. Hillion; V.A. Maraloiu; I.D. Vlaicu; M. Stefan; D. Ghica; H. Rositi; F. Chauveau; M.-G. Blanchin; M. Wiart; V. Dupuis - Ferritin surplus in mouse spleen 14 months after intravenous injection of iron oxide nanoparticles at clinical dose; Nano Research; 10.1007/s12274-016-1126-6 ; (2016) doi: 10.1007/s12274-016-1126-6
Valentin-Adrian Maraloiu
Researcher II
National Institute of Materials Physics
Laboratory of Atomic Structures and Defects in Advanced Materials
405A Atomistilor str., PO Box MG. 7
Magurele - Bucharest, Romania
077125
Telephone: +40212418241
Fax: +40213690177
PROJECTS/ NATIONAL PROJECTS
Copyright © 2026 National Institute of Materials Physics. All Rights Reserved