High energy efficient permanent magnets without rare-earth elements
Project Director: Dr. Palade Petru
National Institute of Materials Physics
The project aims at producing, characterizing and optimizing the magnetic properties of a new class of permanent magnets with high energy efficiency based on iron nitride Fe16N2 with martensite structure. Theoretical predictions for this permanent magnet indicate a maximum energy product (BH)max of up to twice the theoretically maximum allowed for the highest performance magnet up to date, namely Nd2Fe14B. The project addresses a theme that became an imperative of global research and development activities taking into account the tremendous increase of the price of rare earths. It follows a multi-disciplinary approach to the problem. Theoretical calculations based on density functional theory are used for choosing optimal doping elements (transition metals and non-metals) of Fe16N2 which show a favorable effect on increasing thermodynamic stability and also in magnetic properties improvement, i.e. increase of magnetization (via population of sublattices presenting ferrimagnetic configuration) and anisotropy. Simulations of the Fe16N2 magnetic particles embedded in matrices will allow us directing preparation methods in order to obtain magnetic particle size and morphology suitable for enhanced coercivity and high remanence magnetization. Several preparation routes will converge on the obtaining of the compound Fe16N2 and other similar. A first route of preparation uses wet chemical methods that allow obtaining Fe16N2 doped particles. Firstly, it will be obtained the iron oxide or iron oxy-hydroxide precursor with controlled morphology and size using different chemical methods in solution. Subsequently, by thermal treatments of the iron oxide or oxy-hydroxide precursors in hydrogen and ammonia atmosphere one gets Fe16N2 fine magnetic particles with needle-like or ellipsoidal shape that show an important shape anisotropy and high coercivity. The second procedure is to obtain nanocomposites based on Fe16N2 by ball milling the iron powders and doping elements under hydrogen and nitrogen/ammonia reactive atmosphere. Processing of the milled composites and Fe16N2 magnetic particles doped with transition metals and non-metals will be performed using a glove box with controlled atmosphere in order to avoid the exposure to oxygen and moisture from air. Procedures for mixing with binder, orientation in applied magnetic field, pressing and sintering for long time at temperatures below 200 degC will allow to obtain anisotropic permanent magnets based on Fe16N2 with high coercivity and remanence magnetization. The energy product of this magnet will be higher than that of cheap magnets that do not contain rare earth. The magnetic particles and final sintered magnets will by characterized by X-Ray diffraction, neutron diffraction, electron microscopy. Iron-containing phases will be analyzed by Mossbauer spectroscopy. A complex characterization of the magnetic properties (hysteresis, saturation and remanence magnetization, coercivity) will be performed. Optimization of the magnetic properties will assume a permanent feedback between preparation methods – structural / compositional characterization – magnetic properties. The magnets will be coated against corrosion. The new innovative technologies used to produce these magnets will be the subject of patent application. The main outcome of the project, after performing the project activities, will be the permanent magnet without rare earth, which has higher energy product than cheap commercial magnets. A part of the results, which are not subject to patenting, will be disseminated through ISI publications and communications at international conferences.
Main objective: Creating a new class of high efficiency permanent magnet based on iron nitride Fe16N2 with martensitic structure. The approach is multidisciplinary, involving theoretical calculation about finding doping elements with a favorable effect on improving thermodynamic stability and magnetic properties, production of materials required by a variety of preparation methods (chemical synthesis in solution and grinding in ball mill reactive atmosphere) and complex structural, morphological and magnetic characterization.
Specific Objectives:
O1: Calculations of electronic structure of Fe16N2 doped with transition metals and non-metals for the identification of the structures with high thermal stability and favorable magnetic properties for efficient permanent magnets
O2: Preparation and complex characterization of Fe16N2 particles (eventually modified by transition metals and non-metals addition) obtained by wet chemical methods which show suitable magnetic properties to be used for an efficient permanent magnet
O3: Preparation and complex characterization of Fe16N2 composites with addition of transition metals and non-metals produced by ball-milling in reactive atmosphere which exhibit suitable magnetic properties to be used for an efficient permanent magnet
O4: The realization of an innovative technology for production of permanent magnets based on Fe16N2 modified with metals and non-metals by orientation/magnetization in applied magnetic field followed by pressing and sintering
O5: Dissemination of the results through ISI publications and communications at international conferences and patenting the results with the highest applicative potential
PALADE PETRU - Project Director
KUNCSER VICTOR - Senior Researcher 1, Key person
BIRSAN ANCUTA - PhD student, Key person
VALEANU MIHAELA - Senior Researcher 1, Key person
SCHINTEIE GABRIEL - Postdoctoral, Key person
COMANESCU CEZAR CATALIN - Postdoctoral, Key person
MERCIONIU IONEL FLORINEL - Postdoctoral, Key person
MATEI ELENA - Senior Researcher 3
PREDA NICOLETA ROXANA - Senior Researcher 3
GRECULEASA (SANDU) SIMONA GABRIELA - PhD student
KUNCSER ANDREI CRISTIAN - Asistant Researcher
LECA AUREL - Engineer
CIOCA EUGEN MIHAIL - Engineer
PLAPCIANU CARMEN GABRIELA - Senior Researcher
BARTHA MARIA CRISTINA - Senior Researcher 3
FEDER MARCEL - Senior Researcher 1
DIAMANDESCU LUCIAN - Senior Researcher 1
SIMA MARIAN - Senior Researcher 2
SIMA MARIANA - Senior Researcher 3
BURDUSEL MIHAIL - PhD student
GHEORGHE GHEORGHE - Technician
STOICA ANDREI - Technician
ION EMIL - Technician
RUIU GEORGE DANIEL - Technician
HARJOI VICTOR - Worker
Partner 1 - Babes- Bolyai University from Cluj Napoca
BENEA DIANA ANCUTA - Research team leader
POP VIOREL - Prof. Dr., Key person
DEAC IOSIF GRIGORE - Conf. Dr., Key person
MICAN SEVER - Postdoctoral, Key person
TETEAN ROMULUS - Researcher
COLDEA MARIN -Researcher
RADU TEODORA - Researcher
HIRIAN RAZVAN - PhD Student
GAVREA RADU - Master Student
BALASZ IZABELA - Technician
MURESAN VASILE - Technician
Partner 2 - Small and Medium Entreprise - Purtech S.R.L.
CARJAN GHEORGHE CORNEL - Research team leader
MIHALACHE DRAGOS - Dr. Eng. , Key person
MOTIOC SIMION - Engineer , Key person
IORDACHESCU ILINCA - Assistant manager
NISTOR GABRIELA - Financial responsible
scientific report 2014 (in Romanian)- Raport_stiintific_Etapa1_MAGNEF_cod_0971_contract_275_2014_1
scientific report 2015(in Romanian)- Raport_stiintific_tehnic_MAGNEF_Etapa_2_an_2015
scientific report 2016 (in Romanian)-Raport_stiintific_si_tehnic_Et3_2016_MAGNEF_275_2014
scientific report 2017 (in Romanian)-Raport stiintific si tehnic etapa 4 an 2017 MAGNEF PCCA2013 contract 275_2014
final report of the project (in Romanian)-Raport final MAGNEF PCCA2013 contract 275_2014
Main results from 2014
1. Literature review concerning compositions and preparation routes which will be used to prepare Fe16N2 magnetic particles with improved properties
2. Studies concerning optimization of the compositions and preparation routes which will be used to prepare bonded magnets based on Fe16N2 particles
3. Wet chemistry preparation methods and structural investigation of the oxyhydroxides with needle-like morphology

(i) Goethite obtained by controlled oxidation of Fe2+ salts
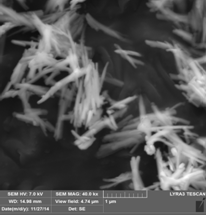
(ii) Goethite obtained from FeNO3/ KOH with molar ratio Fe3+:OH- of 1:6

(iii) Goethite obtained from FeNO3/KOH with molar ratio Fe3+:OH- of 1:12
Main results from 2015
Calculations were performed using the SPRKKR code for evaluation of the magnetic moments for Fe16N2 doped with Ti. Fe substitution with Ti increases the thermodynamic stability of Fe16N2 but slightly reduces magnetic properties.
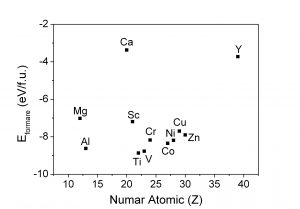
(i) Energy of formation for Fe15XN2 compound (X = metal)
Calculations of energy bands with WIEN2k program showed increased thermodynamic stability of Fe16N2 for the substitution of Fe with Ti or V, among many possible iron substution with metals, and these predictions allow the design of future preparation in order to increase the thermodynamic stability of Fe16N2.

(ii) monodisperse hematite nanoparticles obtained by hydrothermal route


(iii) as prepared iron oxyhydroxide obtained by microwave route
(iv) hematite obtained by annealing in air at 400 0C of iron oxyhydroxide by microwave route)
using heat treatment of iron salts solutions in microwave field were obtained amorphous particles of iron oxyhydroxide which turns in crystalline hematite particles by further annealing in air at 400 0C.
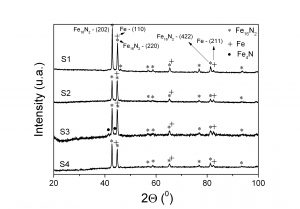
(v) X-ray diffraction data for S1 (goethite nitrided at 140 0C), S2 (hrmatite nitrided at 140 0C), S3 (goethite nitrided at 150 0C) and S4 (processed goethite nitrided at 140 0C)
By treating goethite particles in H2 / Ar flow at 450 0C and then nitriding in ammonia flow at lower temepratures was possible to obtain a significant amount of Fe16N2 and small amount of metallic iron phase.
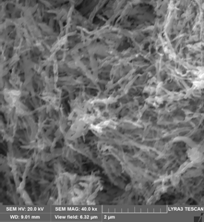
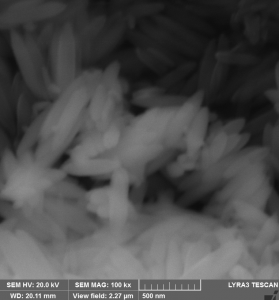
(vi) SEM image of goethite reduced and then nitrided
The Fe16N2 particles retain the needle-like structure of the goethite precursor even after the reduction in H2 / Ar flow at 450 0C and subsequent treatment in ammonia flow.
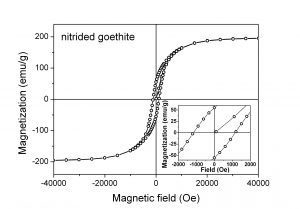
(vii) hysteresis loop at ambient temperature of nitrided goethite
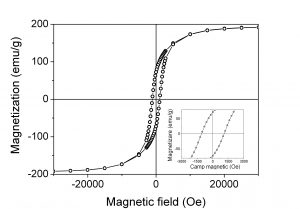
(viii) hysteresis loop at ambient temperature of nitrided goethite pressed in magnetic field
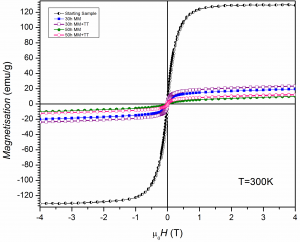
Preliminary permanent magnets were produced by compressing and heating Fe16N2 particles in magnetic field. There is an increase of the remanence from 29% for the original particles up to 38% for the particles oriented and pressed under magnetic field while saturation magnetization (Ms) and coercive field (Hc) remain approximately constant.
Main results from 2016
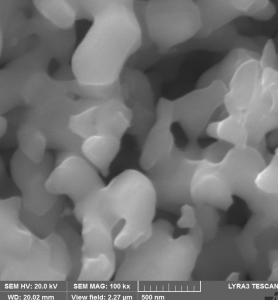
1. Preparation of elongated particles of β-FeOOH (akaganeite) by wet chemistry which were afterwards reduced in 5%H2/Ar and nitrided in ammonia flow
(A) (B)
(i) SEM images of β-FeOOH (akaganeite): as prepared (A) and reduced and nitrided (B)
2. Preparation of transition metal substituted α-FeOOH (goethite) acicular particles by wet chemistry which were afterwards reduced in 5%H2/Ar and nitrided in ammonia flow
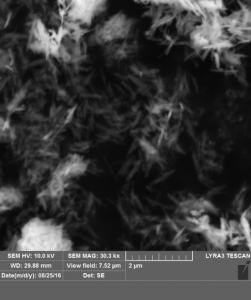
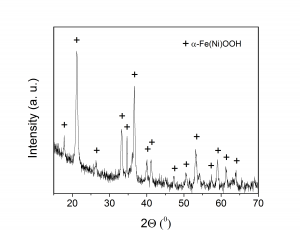
(A) (B)
(ii) SEM image (A) and XRD pattern (B) of α-Fe(Ni)OOH (goethite) with 5 at% Ni
3. Preparation of transition metal substituted amorphous iron oxide by microwave route. After reduction and subsequent long time nitriding it was obtained a mixture or Fe16N2 and Fe
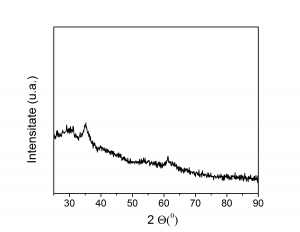
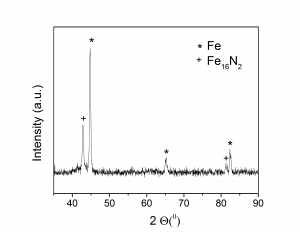
(A) (B)
(iii) XRD spectra of 5at%Ni substituted amorphous iron oxide prepared by microwave route: (A) as prepared, (B) after reduction and subsequent long time nitriding
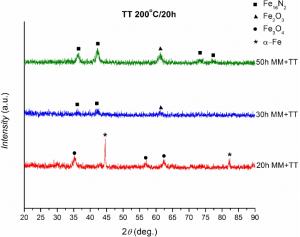
(iv) XRD spectra for Fe16N2 milled composites after various milling time and subsequent annealing
(v) Hysteresis loops for the milled composites containing Fe16N2. Above is presented the full range, while below is shown the central part with higher magnification
It can be observed that the magnetization of saturation decreased strongly after milling and it has values much lower than that of nitrided goethite. However, the coercive field is higher for the composites milled long time than that of nitrided goethite.
5. Based on Mossbauer spectroscopy data we obtained the type of phases (even amorphous or poorly crystallized with superparamagnetic behaviour) and the relative amount of each phase. The samples obtained via microwave route were reduced at 420 0C and subsequently nitrided at various temperatures. At a nitridation temperature of 130 0C was formed mainly metallic iron while above 150 0C, besides the main Fe16N2 phase, appeared also Fe4N and Fe3N with detrimental effect on magnetic properties. The Fe16N2 is characterized by 3 magnetic sextets with hyperfine magnetic fields of about 40.25 T pentru Fe(4d), 31.55 T pentru Fe(8h) si 29.65 T for Fe(4e) crystallographic positions. The other sextets were assigned to Fe, Fe3N and Fe4N, while the central doublet and singlet were assigned to superparamagnetic iron oxide and superparamagnetic iron nitride, respectively.
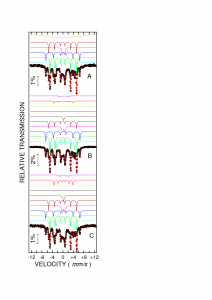
(vi) Mossbauer spectra of samples obtained via microwave route and reduced and subsequently nitrided at various temperatures: 140 0C (A), 150 0C (B), 160 0C (C).
6. The goethite was reduced at various temperatures and subsequent nitrided. At low reduction temperatures were obtained also other Fe-N phases besides Fe16N2 which decrease the total magnetization at saturation
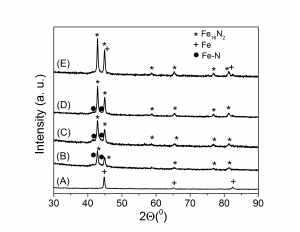
(vii) (A) - goethite reduced at 350 0C, (B) - goethite reduced at 355 0C and nitrided at 140 0C, (C) - goethite reduced at 360 0C and nitrided at 135 0C, (D) - goethite reduced at 385 0C and nitrided at 140 0C, (E) - goethite reduced at 425 0C and nitrided at 140 0C
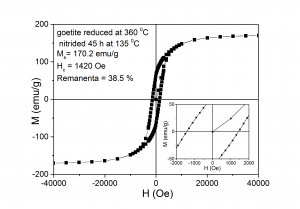
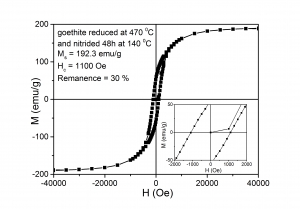
7. The maximum coercivity was obtained for samples reduced at low temperatures while the maximum magnetization at saturation was obtained for samples reduced at high temperatures
(A) (B)
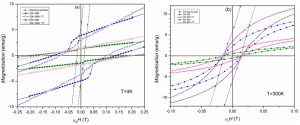
(viii) Hysteresis loops measured at ambient temperature for samples: (A) - reduced at 360 0C and nitrided at 135 0C, (B) - reduced 470 0C and nitrided at 140 0C
8. Goethite reduced at 470 0C and nitrided at 140 0C was pressed (0.8 GPa) perpendicular to applied field (0.3 T). There is a high difference between the remanence measured in plane of the sample and that one measured perpendicular to the plane of the sample
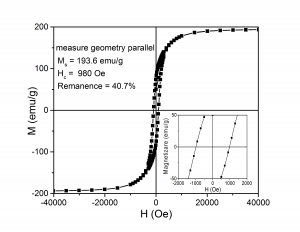
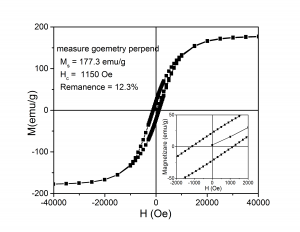
(A) (B)
(ix) Hysteresis loops measured at ambient temperature for goethite pressed perpendicular to magnetic field and measured with the magnetic field applied: (A) - parallel to the plane of the sample, (B) - perpendicular to the plane of the sample
9. By mixing the milled composites with epoxy resin and solidificate them in magnetic field there is a significant difference between the remanence measured for magnetic field applied parallel and perpendicular to the sample during solidification
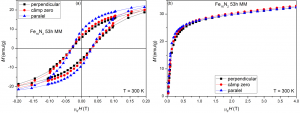
(x) Hysteresis loops and magnetization curves measured at ambient temperature for milled composites mixed with epoxy resin and solidificated in magnetic field oriented parallel and perpendicular to the sample during solidification
Main results from 2017
1. Starting from Fe:Zr powders with mass ratio of 19:1 and by mixing Fe, Zr, Fe4N powders with mass ratio 15.2:0.8:2 were prepared milled composites using a planetary mill in order to see the substitution of Fe by Zr. The reason for this substitution is that, according to first principles simulations, by replacing 1 atom of Fe from Fe16N2 with Ti, Zr or V the stability of Fe16N2 phase increases. The parameters used for miling: 4 h and 8 h miling time, ball to powder ratio 10:1. The samples were subsequent heat treated in 5%H2/Ar flow at 450 0C and nitrided under nitrogen and ammonia flow at temperatures between 160 0C - 300 0C
2. Mossbauer spectroscoy and XRD data proved that Fe16N2 phase was not formed for the milled composites heat treated under ammonia and nitrogen flow. At 220 0C a very low amount of Fe-N phase (not Fe16N2) was detected in the sample annealed in ammonia. Magnetic measurements proved soft magnetic behaviour.
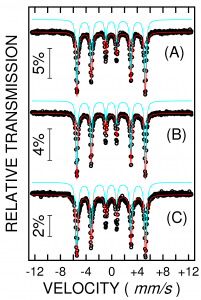
Mossbauer spectra for Fe-Zr milled composites heat treated under ammonia flow ( A - not heated , B - heated at 160 0C , C - heated at 220 0C)
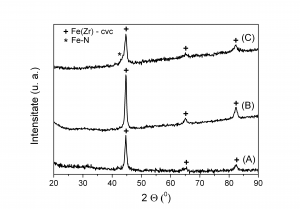
X-Ray diffractograms for Fe-Zr milled composites heat treated under ammonia flow ( A - not heated , B - heated at 160 0C , C - heated at 220 0C)
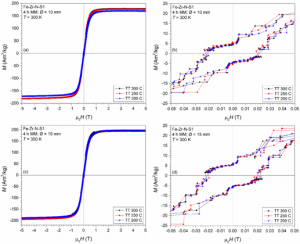
Ambient temperature hysteresis loops for Fe-Zr milled composites heat treated under nitrogen proving soft magnetic behaviour
3. Fe16N2 was prepared by a new route starting from goethite (alpha-FeOOH) produced by controlled oxidation of Fe2+ iron salt solution. For this purpose, deoxygenated aqueous solutions of Na2CO3 and FeSO4 . 7H2O were mixed and immediately afterwards a controlled air flow (2-4 L/min) passed through reactant solutions for 2 - 4 h at temperatures between 40 - 60 0C. Acicular goetite particles with various morphology depending on reaction conditions were obtained by this route.

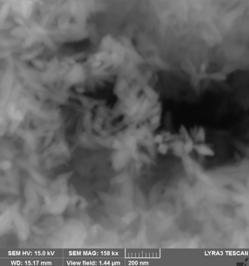
(A) (B)
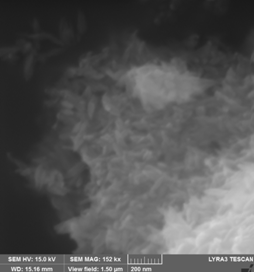
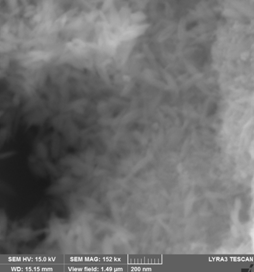
(C) (D)
SEM images for goethite samples prepared starting from FeSO4/Na2CO3 aqueous solutions and heat treated in air flow: (A) - 4h/40 0C, (B) - 8h/40 0C, (C) – 2h/40 0C, (D) – 4h/ 60 0C . Suitable morphology and dimensions in order to get Fe16N2 with good magnetic properties are obtained for (A) - 4h/40 0C
4. Fe16N2 was obtained from goethite produced by Fe2+ carbonated route by reducing under 5% H2/Ar flow at 350 - 450 0C and nitridation under ammonia flow at 140 - 150 0C. The amount of Fe16N2 is very low for sample reduced at 450 0C and afterwards nitrided at 143 0C due to the large Fe grains difficult to nitridate. The samples reduced at lower temperatures are easy to nitridate.
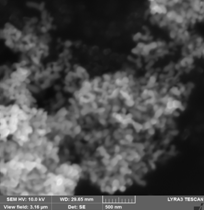
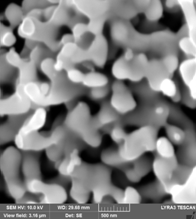
(A) (B)
SEM images for Fe16N2 produced from goethite via Fe2+ carbonated route subsequently processed: (A) - reduced 3h/ 380 0C and nitrided 25h / 143 0C, (B) - reduced 3h/ 450 0C and nitrided 25h / 143 0C
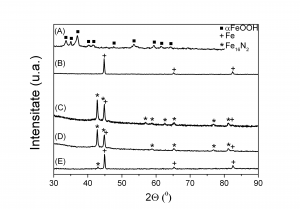
X-ray diffractograms for goethite via Fe2+ carbonated route subsequently processed: (A) - not heat treated, (B) - reduced 3h/360 °C, (C) – reduced 3h/380 °C and nitrided 25h/143 °C, (D) - reduced 3h/390 °C and nitrided 25h/143 °C, (E) – reduced 3h/450 °C and nitrided 25h/143 °C
The sample obtained by reducing at 450 0C and nitriding at 143 0C the goethite obtained via Fe2+ carbonated route exhibits soft magnetic behaviour (low amount of Fe16N2) whereas the sample reduced at 360 0C and nitrided at 143 0C (high amount of Fe16N2) shows much higher coercivity and remanence
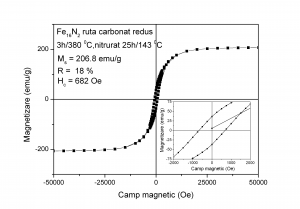
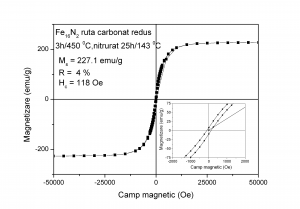
Hysteresis loops measured at ambient temperature for: (A)- sample reduced at 380 0C and nitrided at 143 0C (B) - sample reduced at 450 0C and nitrided at 143 0C
5. Starting from goethite synthesized by coprecipitation from KOH and Fe(NO3)3.9H2O was prepared Fe16N2 by reducing and nitridation. Fe16N2 nanoparticles obtained by this route have magnetization at saturation Ms=193 emu/g, coercivity Hc= 1120 Oe and remanence R=29.3 %. Paralelipipeds (length= 8 mm, width= 2 mm) were produced starting from this powder using a nonmagnetic mold built specially for this project.


(A) - stainless stell nonmagnetic mold for pressing almost paralelipipedic shapes in magnetic field starting from Fe16N2 powder
(B) almost paralelipipedic Fe16N2 magnet
6. The paralelipipedic magnets pressed in magnetic field were annealed under nitrogen flow at temperatures between 150 - 200 0 C for sintering purpose. The cross section SEM images show that grains remain elongated after pressing in magnetic field, but after sintering at 200 0C the structure becomes more compact
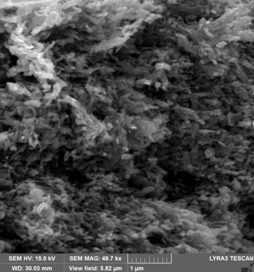
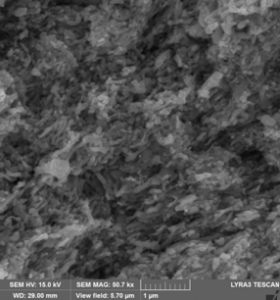
(A) (B)
Cross section SEM images for paralelipipedic magnets from Fe16N2 (goethite reduced and nitridated) powder pressed in magnetic field in paralelipipedic shapes: (A) - without subsequent annealing, (B) annealed under nitrogen flow at 200 0C.
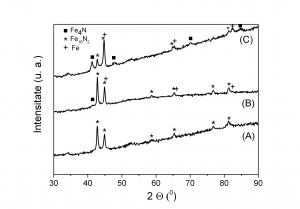
X-ray diffraction data for paralelipipedic magnets obtained by pressing Fe16N2 in magnetic field and subsequent annealing under nitrogen flow: (A) - not annealed, (B) - annealed at 170 0C , (C) - annealed at 200 0C.
The sample not annealed contains only Fe16N2. By annealing at 170 0C the phase Fe4N begins to form. After annealing at 200 0C the main part of Fe16N2 decomposes, forming Fe4N and metallic iron. This behaviour is supported also by Mossbauer spectroscopy measurements
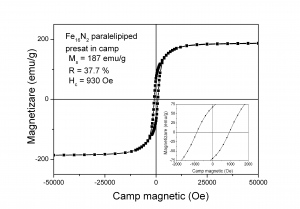 (A)
(A)
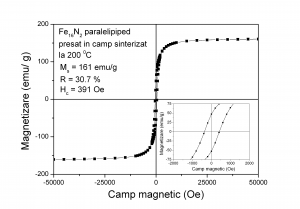 (B)
(B)
Hysteresis loops measured at ambient temperature for the Fe16N2 paralelipipeds pressed in magnetic field and subsequent processed: (A) - not sintered, (B) - sintered at 200 0C.
The magnetic field is applied during measurement in the plane of the paralelipipedic sample (on the direction of the magnetic field used to orientate the acicular nanoparticles during pressing and perpendicular to the pressing direction)
After sintering at temperatures 170 - 200 0C, magnetization at saturation Ms, coercivity Hc and remanence R decrease compared with the not sintered sample due to formation of Fe4Nphase
The remanence bot for the not sintered sample and for the sample sintered at 200 0C increased compared with the remanence of not pressed powder due to shape anisotropy.
NATIONAL PATENTS:
OSIM PATENT REQUEST No. A 00686 from 20.09.2017 entitled (in Romanian)
“Material magnetic pe baza de nanoparticule de nitrura de fier ordonata cu structura martensitica si procedeu de obtinere a lui”
Published ISI articles:
1. “Structural, magnetic and Mossbauer investigation of ordered iron nitride with martensitic structure obtained from amorphous hematite synthesized via microwave route”, Palade, P., Plapcianu, C., Mercioniu, I., Comanescu, C. C., Schinteie G., Leca, A., Vidu, R., Industrial & Engineering Chemistry Research, 56(11) (2017) 2958-2966
2. “Significant change of local atomic configurations at surface of reduced activation Eurofer steels induced by hydrogenation treatments”, Greculeasa, S. G., Palade, P., Schinteie, G., Kuncser, A., Stanciu, A., Lungu, G. A., Porosnicu, C., Lungu, C. P., Kuncser, V., Surface Science, 402 (2017) 114-119
3. “Electronic structure and magnetic properties of the Fe16N2 doped with Ti”, Benea, D., Isnard, O., Pop, V., J. Magnetism and Magnetic Materials 420 (2016) 75-80.
4. “Mossbauer and magnetic investigation of iron nitride with martensite structure synthesized from oxy-hydroxide type precursor”, Palade, P., Plapcianu, C., Mercioniu, I., Comanescu, C. C., Schinteie, G., Digest J. of Nanomaterials and Biostructures 11(1) (2016) 53-63.
5. “Small interfacial distortions lead to significant changes of the half-metallic and magnetic properties in Heusler alloys: The case of the new CoFeZrSi compound”, Birsan A., J. Alloys and Compounds 710(2017) 393-398
6. “Neutron diffraction study of the itinerant-electron metamagnetic Hf0.825Ta0.175Fe2 compound”, Diop, L.V.B., Isnard, O., Suard, E., Benea, D., Solid State Communications229 (2016)16-21.
7. "Cross over between ferro and antiferromagnetic order in Fe itinerant electron magnetism: An experimental and theoretical study of the model (Hf,Ta)Fe2 Laves phases”, Diop, L.V.B., Benea, D., Mankovsky, S.,Isnard, O., J. of Alloys and Compounds 643 (2015) 239–246.
Published BDI articles:
1. “Fe2O3 particles as precursors for α”-Fe16N2 phase synthesis”, One, R.A., Bortnic, R., Mican, S., Barbu-Tudoran, L., Pop, V., Studia Univ. Babes-Bolyai, Physica 61(1) (2016) 93-98.
2. "Surfactant effect on the structural and magnetic properties of Fe powders prepared by wet milling", Hirian, R., Mican, S. Neamtu, B., Pop, V., Studia Univ. Babes-Bolyai, Physica, 60 (2) (2015) 53-58.
Book chapter:
1. “Transition metal/ Carbon Nanocomposites” in Carbon Nanomaterials Sourcebook: Nanoparticles, Nanocapsules, Nanofibers, Nanoporous Structures and Nanocomposites, vol II, V. Kuncser, P. Palade, G. Schinteie, F. Dumitrache, C. Fleaca, M. Scarisoreanu, I. Morjan, G. Filoti, p. 597-619, CRC press, K. D. Sattler eds., (24 March 2016), 724 pages, ISBN 9781482252705.
International conferences:
1. “Hard Magnetic Materials: Present and Perspectives”, Pop, V., invited lecture at International Conference on Powder Metallurgy & Advanced Material (RoPM-AM 2017), 17-20 september 2017 - Cluj-Napoca, Romania
2."Synthesis, structural, electronic and magnetic properties of α”-(Fe1-xMx)16N2 (M = Ti, Zr)", One R.,A., Mican S., Benea, D., Pop, V., poster presented atThe 11th International Conference on Physics of Advanced Materials (ICPAM 2016), 8-14 september 2016 - Cluj-Napoca, Romania.
3. "Electronic Structure and Magnetic Properties of the Fe16N2 doped with Ti", Benea, D., Isnard, O., Pop, V., oral presentation at The 8th International Conference on Advanced Materials (ROCAM 2015), 7-10 july 2015, Bucharest.
PROJECTS/ NATIONAL PROJECTS
Copyright © 2025 National Institute of Materials Physics. All Rights Reserved