Biologically active gold substrates and nanoparticles by grafted polyhydrazide scaffolds for high-throughput screening applications – BIOGOLDSURF
Project Director: Dr. Daniel CRISAN
Biologically active gold substrates and nanoparticles by grafted polyhydrazide scaffolds for high-throughput screening applications
Acronim: BIOGOLDSURF
Project Code/Cod proiect: PN-III-P1-1.1-PD-2019-0100
Contract number/Numar contract: PD53 din 2020
Project Director/Director de proiect: Dr. Daniel Nicolae Crisan
Financed by/Finantat de: Unitatea Executiva pentru Finantarea Invatamantului Superior, a Cercetarii, Dezvoltarii si Inovarii, UEFISCDI
Contracted by/Contractat de: Institutul National de Cercetare-Dezvoltare pentru Fizica Materialelor (INCDFM)
Status: Finished/Finalizat
Starting date/Data inceperii: 1 septembrie 2020
End date/Data finalizarii: 30 noiembrie 2022
Project Summary:
This project aimed to develop a platform for obtaining gold surfaces grafted with functional polymers. To achieve this goal, it was proposed to graft gold surfaces with polyhydrazide scaffolds and exploit their post-polymerization modification chemistry to impart surfaces biological activity for high-throughput screening. The main challenge the project proposed to address is the thorough characterisation of surfaces and interfaces in order to understand and prove the functionalisation of grafted polymers.
The recent technological advances in the methods for surface characterisations are expected to be a cornerstone for materials with biological applications. Biotechnologically advanced materials can be obtained by grafting polymers onto various substrates in order to modify the interface between the surface and its external environment. Properties at the interfaces (i.e. substrate strips or nanoparticles), such as adhesion, wettability, chemical reactivity and biocompatibility can be tuned through rational designs of grafted polymer layers. Substrates can be imparted with protection against corrosive and radiant energy damage, variable hydrophobicity, enhanced or diminished interaction with cells or biomolecules, and even instilled with bactericidal or bacteriostatic properties. In the case of nanoparticles, grafted polymers can also be used to control the size of the particles, prevent aggregation, and provide and control the optical properties.
In the context of biologically active surfaces for high-throughput screening, the synthesis of libraries of gold substrates and NPs with grafted polymers can be time consuming, expensive, and synthetically challenging due to the large number of functional polymers that need to be synthesized, characterised and grafted. To address this issue, this project proposed to employ post-polymerization modification of grafted polymers to facilitate the discovery and development process for materials with tuneable biological properties.
Project Objectives
- To synthesize tert-butyl 2-acryloylhydrazinecarboxylate and tert-butyl 2-(4-ethynylbenzoyl)hydrazine-1-carboxylate and polymerize these monomers via RAFT polymerization and metal catalyzed polymerization respectively.
- To use different grafting strategies to make polymers-grafted Au surfaces on gold substrates and gold nanoparticles.
- To use post-polymerization functionalization on grafted polymers to obtain gold surfaces with biological applications.
Substraturi si nanoparticule de aur cu activitate biologica obtinute prin grefare cu polihidrazide
Rezumatul proiectului:
Proiectul a avut ca scop dezvoltarea unei platforme pentru obtinerea suprafetelor de aur grefate cu polimeri functionali. Pentru a indeplini acest obiectiv, am propus grefarea suprafetelor de aur cu polimeri schela, de tip polihidrazide, si exploatarea functionalizarii chimice post-polimerizare pentru a imprima suprafetelor activitate biologica. Principala provocoare a fost reprezentata de caracterizarea amanuntita a suprafetelor si interfetelor pentru a intelege si a demonstra functionalizarea polimerilor grefati.
Dezvoltarile tehnologice recente in domeniul metodelor de caracterizare a suprafetelor poate reprezenta fundatia pentru o generatie noua de materiale cu aplicatii biologice. Materialele avansate biotehnologice pot fi obtinute prin grefarea de polimeri pe diferite tipuri de substraturi pentru a modifica interfata intre suprafata si mediul exterior. Proprietatiile de la interfata, precum aderenta, caracterul hidrofilic, reactivitatea chimica sau biocompatibilitatea pot fi ajustate prin modelarea planificata a straturilor polimerice grefate. Suprafetele pot fi modificate pentru a imbunatati sau a diminua interactiunea cu celule sau biomolecule si inclusiv pentru a reda proprietati bactericide sau bacteriostatice. In cazul nanoparticulelor de aur, prin grefarea polimerilor se poate controla marimea particulelor, se poate preveni agregarea si se pot controla proprietatile optice.
Referitor la suprafetele active biologice pentru accelerarea procesului de cercetare si dezolvatare, sinteza substraturilor si nanoparticulelor de aur grefate cu polimeri poate fi un mare consumator de timp, resurse materiale, economice si sintentic problematic din pricina numarului mare de polimeri functionali necesari pentru o investigare amanuntita. Pentru a diminua impactul acestor etape, am propus ultilizarea procesului de functionalizare post-polimerizare a polimerilor grefati pentru a accelera procesul de sinteza si dezvoltare a materialelor cu proprietati biologice optimizabile.
Obiective
- Sinteza monomerilor de tip tert-butil acriloilhidrazinacarboxilata si a tert-butil 2-4(etinilbenzoil)hidrazina-1-carboxilata si polimerizarea lor prin polimerizare de tip RAFT sau prin polimerizare catalizata de metale, fie in solutie, sau direct pe suprafete modificate cu aur.
- Prepararea de substraturi de aur si nanoparticule de aur grefate cu polimeri de tip polihidrazide prin metoda grefarii de la suprafata, pe suprafata, si grefarea in-situ.
- Pentru a demonstra principiul folosirii acestor suprafete de aur grefate cu polihidrazide in domeniul biologic, se va utiliza modificarea post-polimerizare a hidrazidelor pentru a functionaliza suprafetele in vederea immobilizarii biomoleculelor sau a gruparilor chimice ce pot interactiona cu acestea.
WORKING PLAN
Objective 1: Monomer and Polymer synthesis
Act. 1.1: Monomer synthesis - Synthesis and characterisation of tert-butil 2-acryloylhydrazinecarboxylate and tert-butil 2-(4-ethynylbenzo)hydrazine-1-carboxylate
Act. 2.2: Polymer synthesis - RAFT polymerisation of tert-butil 2-acryloylhydrazinecarboxylate and metal catalyzed polymerisation of tert-butil 2-(4-ethynylbenzo)hydrazine-1-carboxylate
Objective 2: Preparation of grafted surfaces
Act. 2.1: Preparation of substrates and nanoparticles - In this activity, surfaces on substrate strips will be prepared by sputtering, vacuum thermal evaporation or chemical deposition methods, and nanoparticles will be synthesized via the reduction of chloroauric acid using sodium citrate as a reducing and capping agent.
Act. 2.2: Anchoring of initiators and CTA on surfaces - Surfaces prepared will be chemically modified firstly with anchoring groups such as functional thiols, that will be further modified to present a CTA or a polymerization initiator. The amount of functional anchors at the surface will be varied in order to influence the grafting density and morphology during the grafting-from process.
Act. 2.3: "In-situ" grafting of Au NPs - This activity is concerned with the formation of grafted Au NPs by the “in-situ” method. Poly(acryloyl hydrazide) capped with thiols will be mixed at different concentrations with Au salts that will be reduced to afford Au NPs grafted with poly(acryloyl hydrazide). The effect of the polymer chain length and concentration on the size of the Au NPs will be investigated.
Act.2.4: Preparation of grafted-to surfaces - Surfaces prepared in act. 2.1. will be chemically modified with polymers synthesized in act. 1.2. capped with thiols for binding to gold. Grafting density and morphology of the polymer brushes will be investigated as a function of polymer chain length and concentration of polymer.
Act. 2.5: Preparation of grafted-from surfaces - Anchored surfaces from A2.2. will be grafted by carrying out polymerization reactions with the surfaces submerged or dispersed in the reaction solution. The grafting density will be investigated as a function of anchored species at the surface. Different brush thickness will be obtained by controlling the controlled polymerization conditions.
Objective 3: Functionalization and applications
Act. 3.1: Functionalization with phenylboronic acid derivatives - In this activity, grafted surfaces will be functionalized with phenylboronic acid derivatives. This functional group can bind carbohydrate groups such as those in yeast cell walls (with highly mannosylated proteins) or glycoproteins (antibodies).
Act. 3.2: Polylisine arrays for single cell analysis - Surfaces will be functionalized with polylisine (or other positively charged molecules) for the immobilization of yeast cells (having negatively-charged wall) prone to single cell analysis.
Act. 3.3: Amphiphilic polymers for oligonucleotide binding - this activity, grafted Au NPs will be functionalized with guanidinium groups and different hydrophobic aldehydes.
PLAN DE REALIZARE
Obiectivul 1: Sinteza monomerilor si a polimerilor
Act. 1.1: Sinteza monomeri - Sinteza si caracterizarea a tert-butil 2-acriloilhidrazinacarboxilat si a tert-butil 2-(4-etinilbenzoil)hidrazina-1-carboxilat.
Act. 1.2: Polimerizarea monomerilor - Polimerizarea RAFT a tert-butil 2-acriloilhidrazinacarboxilat si polimerizarea catalizata de metal a 2-(4-etinilbenzoil)hidrazina-1-carboxilat.
Obiectivul 2: Preparea de substraturi si nanoparticle de aur grefate cu polihidrazide
Act. 2.1: Prepararea substraturilor si a nanoparticulelor - Substraturi de aur vor fi preparate prin evaporare termica, pulverizare catodica asistata de magnetron (sputtering) sau depunere chimica. Nanoparticule de aur vor fi preparate prin reducerea chimica a acidului cloroauric.
Act. 2.2: Ancorarea initiatorilor si agentilor de transfer pe sufrafata - Functionalizarea substraturilor cu tioli functionali urmata de functionalizarea cu agenti de transfer sau initiatori de polimerizare.
Act. 2.3: Grefarea "in-situ" a nanoparticulelor de aur - Formarea de nanoparticule de aur grefate prin metoda "in-situ" cu poli(acriloil hidrazida).
Act. 2.4: Prepararea suprafetelor prin grefarea la suprafata - Polimerii pregatiti anteriori vor fi grefati covalent la suprafatele aurite.
Act. 2.5: Preparare a suprafetelor grefate prin metoda grefarii de la suprafata - Substraturile pregatite si modificate anterior vor fi imersate in solutiile reactiilor de polimerizare.
Obiectivul 3: Functionalizarea suprafetelor prin modificarea post-polimerizare a hidrazidelor
Act. 3.1: Functionalizarea cu derivati ai acidului fenil boronic - Suprafete grefate vor fi functionalizate cu derivati ai acidului fenil boronic, grupare ce se poata lega de grupari de tip carbohidrat precum cele in peretii celulelor de drojdie.
Act. 3.2: Retea de tip polilisina pentru analiza celulara - Supfrafetele vor fi functionalizate cu polilisina sau alte molecule incarcate pozitiv in vederea immobilizarii celulelor de drojdie.
Act. 3.3: Polimeri amfifilici pentru conjugarea cu oligonucleotide - Nanoparticule de aur grefate vor fi modificate cu grupari guanidiniu si aldehyde hydrofobe in vederea conjugarii cu oligonucleotide.
Director Proiect: Dr. Daniel Nicolae Crisan (U-1900-062H-0148)
https://www.brainmap.ro/daniel-nicolae-crisan
Mentor Proiect: Prof. Ileana Cornelia Farcasanu (U-1700-033C-8247)
https://www.brainmap.ro/ileana-cornelia-farcasanu
Between 29th August - 3rd September 2021, preliminary results were presented at the 72nd Annual Meeting of the International Society of Electrochemistry -Jeju, South Korea in a poster presentation titled "Hydrazide-based Materials for Carbonyl Detection in Oxidized Proeteins".
June 2022: Article was published. -“Bioconjugates of mercaptocarboxylic acids functionalized AuNP and superoxide dismutase for superoxide electrochemical monitoring”, C. G. Sanz, D. N. Crisan, R. J. B. Leote, M. Onea, M. M Barsan, Mikrochim Acta. 2022, 8, 189 doi: 10.1007/s00604-022-05352-z
Between 24th July - 28th July 2022, preliminary results were presented at the 84th Prague Meeting on Macromolecules - Prague, Czechia in a poster presentation titled "Grafting Strategies for the Synthesis of Polihydrazide-Gold Nanoparticles".
17th October 2022: Patent titled (“Metodă de grefare a nanoparticulelor de aur cu polihidrazide cu aplicatii in bioconjugarea acestora cu enzime” submitted to OSIM for acceptance.
Both the Boc protected and the deprotected derivatives of the targeted monomers were synthesized. As such the hydrochloride salt of acryloyl hydrazide and the TFA salt of 4-ethynylbenzo hydrazide were used in the grafting stages.
S-au sintetizat şi caracterizat cu succes atât formele protejate cu gruparea Boc ale monomerilor propuşi, cât şi formele deprotejate (i.e. hidroclorura de acriloil hidrazida, şi sarea acidului trilfuoroacetic ale 4-etinylbenzohidrazidei) necesare în etapele de grefare.
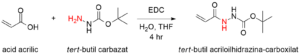
Scheme 1: Reaction scheme showing the synthesis of the Boc-protected monomer tert-butyl 2-acryloylhydrazinecarboxylate.
Schema 1: Reacţia sintezei monomerului projetat tert-butil 2-acriloilhidrazină-carboxilat.

Scheme 2: Reaction scheme showing the synthesis of the deprotected monomer acryloyl hydrazide hydrochloride.
Schema 2: Reacţia sintezei monomerului deprojetat clorhidrat de acriloil hidrazidă.
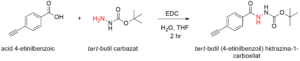
Scheme 3: Reaction scheme showing the synthesis of the Boc-protected monomer tert-butyl 2-(4-
ethynylbenzoyl)hydrazine-1-carboxylate.
Schema 3: Reacţia sintezei monomerului projetat tert-butil 2-(4-etinilbenzoil)hidrazină-1-carboxilat.
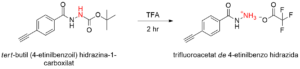
Scheme 4: Reaction scheme showing the synthesis of the deprotected monomer (4-
ethynylbenzoyl)hydrazide trifluoroacetate.
Schema 4: Reacţia sintezei monomerului deprojetat trifluoroacetat de 4-etinilbenzo hidrazidă.
Polymers with varying degrees of molecular weights and dispersities were synthesised and characterised and used after deprotection in the grafting of gold surfaces.
Polimeri cu diferite greutăţi moleculare ale monomerilor protejaţi au fost sintetizaţi şi caracterizaţi, şi folosiţi după deprotejare în procesul de grefare a suprafeţelor de aur.
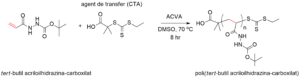
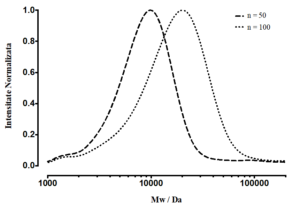
Scheme 5: Reaction scheme showing the RAFT polymerization of the Boc-protected monomer tert-butyl 2-acryloylhydrazinecarboxylate monomer (left). SEC/GPC chromatogram of two batches of poly(tert-butyl 2-acryloylhydrazinecarboxylate) (right).
Schema 5: Reacţia sintezei de polimerizare a monomerului projetat tert-butil acriloilhidrazină-carboxilat, şi cromatogramele SEC/GPC ale polimerului protejat poli(tert-butil acriloilhidrazină-carboxilat)
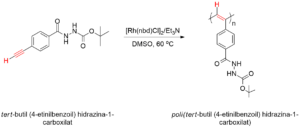
Scheme 6: Reaction scheme showing the metal catalyzed polymerization of the tert-butyl 2-(4-
ethynylbenzoyl)hydrazine-1-carboxylate monomer.
Schema 6: Reacţia sintezei de polimerizare a monomerului projetat tert-butil (4‑etinilbenzoil) hidrazină-1-carboxilat.
Grafting of gold nanoparticles was carried out and evidenced by all three methods with poly(acryloyl hydrazide) and by grafting-to and in-situ grafting with poly(4-ethynylbenzo hydrazide). Grafting from strategies have proven the most complicated method due to the larger number of successive syntheses steps required in the preparation of the surfaces prior to polymerization. These successive reactions lead in time to the loss of the narrow dispersity and spherical shape of the nanoparticles obtained using the initial citrate reduction method. By grafting-to methods, monodisperse and spherical shaped, hydrazide coordinated gold nanoparticules were obtained, without the requirement of thiol capped functional groups. The most important results were obtained following the evaluation of in-situ grafting. In these experiments it was observed that the hydrazide side-chains of the polymer species can act both as reducing agents and as coordinating groups, thus eliminating the need to pre-synthesise the gold nanoparticles using conventional means.
Grefarea nanoparticulelor de aur a fost obţinută şi evidenţiată prin toate cele trei metode de grefare în cazul poli(acriloil hidrazidei) şi prin grefarea la suprafaţa şi in-situ în cazul poli(4-etinilbenzo hidrazidei). Grefarea de la suprafaţa nanoparticulelor de aur s-a dovedit cea mai complicată metodă, din cauza multiplelor reacţii chimice succesive necesare pregătirii suprafeţei pentru polimerizare, reacţii care în timp duc la pierderea monodispersităţii şi sfericităţii nanoparticuelor obţinute prin sinteza clasică a nanoparticulelor de aur prin reducerea cu citrat. Prin grefarea la suprafata s-au obţinut nanoparticule de aur, monodisperse şi sferice, coordinate prin grupările hidrazide, neffind necesare prezenţa grupărilor tiol terminale. Cele mai interesante rezultate s-au obţinut în urma experimentelor de grefare in-situ, unde s-a observat că grupările hidrazide pot servi atât rol de coordinare, cât şi rol de agent reducător a aurului, astfel obţinându-se nanoparticule grefate in-situ cu ambele polihidrazide fără necesitatea sintetizării în prealabil a nanoparticulelor de aur prin metode clasice.
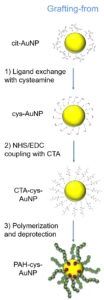
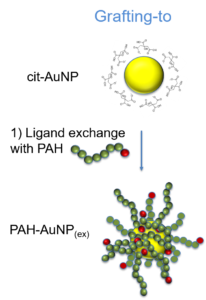
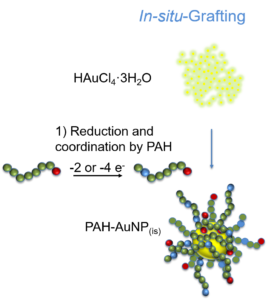
Scheme 7: The three grafting strategies explored during the project.
Schema 7: Strategiile de grefare explorate în cadrul proiectului.
The advtanges of using gold nanoparticles in biosensing devices was explored, at first through the evaluation of difference arising between ionic and covalent binding of an enzyme (superoxide dismutase) at the nanoparticle surface. This showed that long aliphatic ligands such as 11-mercaptoundecanoic acid helps increase the stability of the enzyme while shorter aromatic ligands such as 4-mercaptobenzoic acid increase the sensitivity of the probes by facilitating electron transfer processing through the aromatic rings. By immobilizing alcaline phosphatase enzymes to polyhydrazide grafted nanoparticles, highly stable biosensors were obtained compared to similar nanoparticles grafted with conventional ligands. Furthermore, the post-polymerisation functionalisation of the polyhydrazide-AuNP obtained was evaluated both with biomolecules through their carboxilic acid groups, but also with carbonyl contaning moieties, showing in the process the potential of applying these products in biological applications such as biosensors or cell growth interactions.
S-a explorat avantajul folosirii nanoparticulelor de aur în dispozitive de tip biosenzor, întâi prin evaluarea diferenţelor între conjugarea covalentă versus interactiunea ionică a unei enzime (superoxid dismutază) cu suprafaţa nanoparticulei, şi apoi s-a demonstrat că liganzii alifatici lungi (i.e. acid 11-mercaptoundecanoic) ajută la o stabilizare mai bună a enzimei pe când liganzii scurţi aromatici (i.e. acid 4-mercaptobenzoic) permit o senzitivitate crescută prin capacitatea de transfer de electroni. Prin immobilizarea enzimelor fosfataza alcalină la nanoparticule de aur grefate cu polihidrazide, s-a obţinut un biosenzor cu o stabilitate în timp ridicată comparativ cu nanoparticule stabilizate cu liganzi uzuali. Astfel, s-a dovedit atât potenţialul funcţionalizării post-polimerizare a suprafeţelor de aur grefate cu polihidrazide cu molecule continând grupări carbonil cât şi cu biomolecule prin grupările carboxilice, dar şi faptul că aceste funcţionalizări permit utilizarea acestei metode de grefare şi functionalizare în aplicaţii biologice precum biosenzori sau interacţiuni în cresterea celulelor.
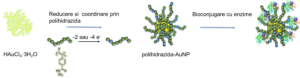
Scheme 8: Enzyme bioconjugation strategies with a view to create a highly localized enzyme concentration with a high stability in time.
Schema 8. Bioconjugare cu enzime in vederea fabricarii unor biosenzori cu concentratie locala ridicata de enzima si stabilitate crescuta in timp.

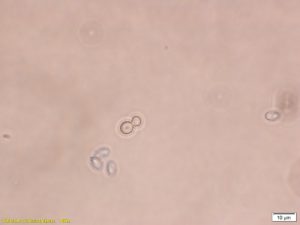


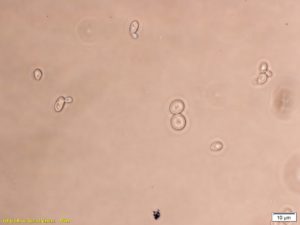

Microscopy images of Saccharomyces cerevisiae cells grown in the presence of polyhydrazide-AuNPs functionalized with different aldehydes.
Imagini Microscopie celule Saccharomyces cerevisiae incubate in prezenta unor nanoparticule grefate cu polihidrazide functionalizate cu diverse aldehide.
As such, through these studies regarding covalent enzyme immobilization, functionalization with different aldehydes and investigating the structure-activity relationship with regards to eucaryotic cell growth and through studies of immobilization of biomolecules using bound phenylboronic acid, it was shown as a proof-of-concept that gold nanoparticles grafted with polyhydrazides can be functionalized to impart potential biomedical applications.
Astfel, din studiile asupra immobilizării covalente a enzimelor, functionalizării cu diferite aldehide şi investigarea relaţiei structură-activitate biologică prin efectul asupra creşterii unor celule eucariote, şi studii asupra immobilizarii prin acidul fenil boronic, s-a demonstrat conceptual că nanoparticulele de aur grefate cu polihidrazide pot fi funcţionalizate în scopul unor potenţiale aplicaţii biomedicale.
-“Bioconjugates of mercaptocarboxylic acids functionalized AuNP and superoxide dismutase for superoxide electrochemical monitoring”, C. G. Sanz, D. N. Crisan, R. J. B. Leote, M. Onea, M. M Barsan, Mikrochim Acta. 2022, 8, 189 doi: 10.1007/s00604-022-05352-z https://link.springer.com/article/10.1007/s00604-022-05352-z#Abs1
Contact
Dr. Daniel Nicolae Crisan
Cercetator Stiintific Gradul IIII
E-mail: daniel.crisan@infim.ro
Departament: Laboratory of Multifunctional Materials and Structures
PROJECTS/ PROIECTE NATIONALE
Copyright © 2025 National Institute of Materials Physics. All Rights Reserved